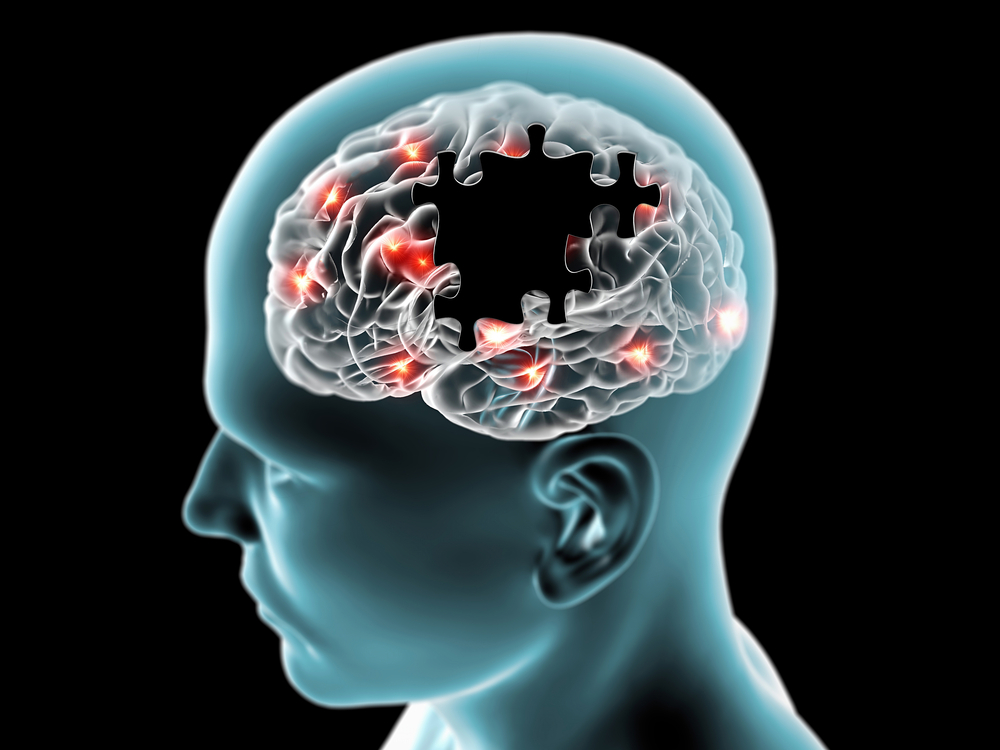
Debilitating tau protein tangles and sticky beta-amyloid plaques inside brain cells have long been linked to Alzheimer’s disease, a neurodegenerative disorder. But a previously unknown culprit—fat droplets—has come to light thanks to current study. These fat deposits inside brain cells may play a major role in the onset and progression of Alzheimer’s disease, according to a ground-breaking research by Michael Haney of the University of Pennsylvania.
Interpreting the Study’s Results
Investigating the APOE gene, a significant genetic risk factor for the illness, allowed Haney and his colleagues to better understand the function of fat droplets in Alzheimer’s. The regulation of fat’s entry and exit from cells is greatly aided by this gene. The many APOE gene variations were of special interest; APOE4 was linked to the greatest risk of Alzheimer’s.
Their study showed that immune cells with the APOE4 mutation released more of an enzyme that encourages fat accumulation in the brains of those patients. Furthermore, scientists found a significant increase in fat storage, especially in cells bearing the APOE4 variation, when they exposed immunological brain cells (microglia) to amyloid, another characteristic of Alzheimer’s disease.
Consequences for Therapy Development and Treatment
The way that Alzheimer’s disease is understood and treated will be significantly affected by these results. The identification of fat droplets as a contributory component offers up new options for therapeutic intervention, since prior efforts have mostly concentrated on targeting protein culprits like tau and beta-amyloid.
According to Haney, more potent Alzheimer’s disease therapies may result from focusing on these fat droplets within brain cells. It could be possible for researchers to slow down or even stop the disease’s course by creating treatments that improve fat metabolism or decrease fat storage.
Including the Fat Droplet Theory in the Knowledge Base
The fact that fat droplets may have a role in Alzheimer’s disease highlights how complicated the condition is. It implies that successful therapy would need a multimodal strategy that targets a number of underlying processes, such as inflammation, protein aggregation, and now fat metabolism.
The research also emphasizes the connections between several cellular processes linked to Alzheimer’s disease. Further evidence for the fat droplet idea comes from the fact that genes linked to a slightly elevated risk of the illness often affect the immune system or fat metabolism.
Concluding Remarks: A Bright Future
An important development in the study of neurodegenerative illnesses is the identification of fat accumulation within brain cells as a causative agent of Alzheimer’s. Researchers are laying the groundwork for more specialized and efficient therapies by deepening our knowledge of the illness causes.
It is becoming more and more obvious that a comprehensive strategy that takes into account all aspects of the illness is necessary as we continue to untangle the complexity of Alzheimer’s. With the potential to improve millions of people’s lives by treating Alzheimer’s disease, the fat droplet hypothesis offers fresh hope for treatment development.
Find out about the ground-breaking research that reveals the involvement of adipose deposits in Alzheimer’s disease and the therapy implications. Examine the possibilities of focusing on these fat droplets to develop more potent treatments.